Table of Contents
ToggleLiquid silicone O-rings play a critical sealing role in modern medical devices. From infusion pumps and ventilators to surgical instruments and diagnostic equipment, medical-grade O-rings must ensure leak-proof performance, biocompatibility, and long-term stability under strict regulatory conditions.
Compared with traditional rubber materials, Liquid Silicone Rubber (LSR) offers superior purity, flexibility, and resistance to temperature, chemicals, and aging—making it the preferred material for medical O-ring production.
In this guide, we will explain how liquid silicone O-rings for medical devices are produced, covering material selection, mold design, injection molding processes, quality control, and regulatory compliance.
2. What Is a Medical-Grade Liquid Silicone O-Ring?
A medical-grade liquid silicone O-ring is a circular elastomeric seal manufactured from platinum-cured liquid silicone rubber and designed specifically for use in medical applications.
Key Characteristics:
- Biocompatible (USP Class VI, ISO 10993)
- Non-toxic, odorless, and tasteless
- Excellent elasticity and compression set resistance
- Stable under sterilization (steam, EtO, gamma)
- High chemical and temperature resistance
These properties make LSR O-rings ideal for dynamic and static sealing applications in medical environments.
3. Why Choose Liquid Silicone Rubber (LSR) for Medical O-Rings?
3.1 High Biocompatibility
Medical LSR is platinum-cured and contains no peroxide byproducts, reducing the risk of cytotoxicity or allergic reactions.
3.2 Excellent Sealing Performance
LSR maintains elasticity over a wide temperature range (-60°C to 200°C), ensuring reliable sealing even under repeated compression.
3.3 Sterilization Resistance
Liquid silicone O-rings withstand:
- Autoclave steam sterilization
- Ethylene oxide (EtO)
- Gamma irradiation
3.4 Long Service Life
LSR resists aging, UV exposure, and chemical degradation, making it ideal for long-term medical device use.

4. Medical Applications of Liquid Silicone O-Rings
Liquid silicone O-rings are used in:
- Syringes and infusion systems
- Dialysis equipment
- Respiratory and ventilator systems
- Catheters and connectors
- Surgical instruments
- Drug delivery devices
In these applications, failure is not an option, which is why material purity and manufacturing precision are essential.
5. Material Selection for Medical Liquid Silicone O-Rings
5.1 Medical-Grade LSR Standards
When producing O-rings for medical devices, the LSR material must comply with:
- ISO 10993 (Biological evaluation)
- USP Class VI
- FDA CFR 21 compliance
- RoHS and REACH (if applicable)
5.2 Shore Hardness Selection
Common hardness ranges:
- Shore A 30–40: Soft sealing applications
- Shore A 50–60: Standard medical O-rings
- Shore A 70+: High-pressure sealing
Selecting the correct hardness ensures optimal compression and sealing performance.

6. Design Considerations for Medical Silicone O-Rings
6.1 Dimensional Accuracy
Medical O-rings require tight tolerances, often within ±0.02 mm, to ensure compatibility with device housings.
6.2 Surface Finish
A smooth surface finish reduces:
- Particle generation
- Friction
- Bacterial adhesion
6.3 Flash Control
Excess flash is unacceptable in medical applications. Precision mold design and optimized injection parameters are critical.
7. Tooling and Mold Design for LSR O-Rings
7.1 Mold Material
High-grade stainless steel (e.g S136) is commonly used to meet cleanliness and durability requirements.
7.2 Cold Runner System
Medical LSR O-rings typically use cold runner injection molds, which:
- Reduce material waste
- Improve consistency
- Maintain material purity
7.3 Multi-Cavity Design
For high-volume production, multi-cavity molds increase efficiency while maintaining dimensional consistency.

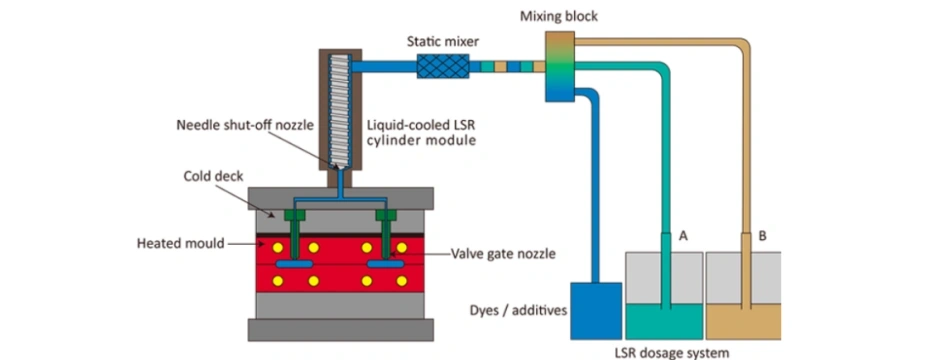
8. Liquid Silicone Injection Molding Process for Medical O-Rings
8.1 Material Preparation
LSR consists of two components (Part A and Part B) mixed in a 1:1 ratio, often with color or additives added during dosing.
8.2 Injection Molding
The mixed LSR is injected into a heated mold cavity where it cures rapidly through platinum catalysis.
8.3 Vulcanization
Curing occurs at temperatures typically between 160°C and 200°C, forming a solid, elastic O-ring.
8.4 Demolding
Medical-grade O-rings are demolded carefully to avoid deformation or contamination.
9. Post-Curing and Secondary Processes
9.1 Post-Curing (Optional)
Some medical applications require post-curing to:
- Remove volatile residues
- Improve mechanical properties
- Meet regulatory requirements
9.2 Deflashing
Precision deflashing methods include:
- Cryogenic deflashing
- Manual trimming (for low volumes)
10. Quality Control for Medical Liquid Silicone O-Rings
10.1 Incoming Material Inspection
- Material certification verification
- Lot traceability
10.2 In-Process Inspection
- Dimensional measurement
- Visual inspection for flash, bubbles, or defects
10.3 Final Testing
Common tests include:
- Compression set
- Tensile strength
- Elongation
- Biocompatibility testing
- Cleanliness and particle testing

11. Cleanroom Manufacturing Requirements
Medical silicone O-rings are often produced in:
- ISO Class 7 or Class 8 cleanrooms
Cleanroom production minimizes contamination and ensures compliance with medical device standards.
12. Regulatory and Compliance Requirements
Key Certifications:
- ISO 13485 (Medical device quality management)
- FDA registration
- Material CoC and CoA
- Full traceability documentation
A qualified LSR manufacturer should provide complete regulatory support for medical customers.
13. Choosing the Right Medical LSR O-Ring Manufacturer
When selecting a supplier, consider:
- Medical LSR experience
- In-house mold design
- Cleanroom molding capability
- ISO 13485 certification
- Long-term supply stability
A reliable partner ensures product safety, regulatory compliance, and cost efficiency.

14. Conclusion
Producing liquid silicone O-rings for medical devices requires precision engineering, medical-grade materials, and strict quality control. From material selection and mold design to cleanroom injection molding and compliance testing, every step impacts performance and safety.
By choosing liquid silicone rubber and working with an experienced medical LSR manufacturer, medical device companies can achieve reliable sealing solutions that meet the highest industry standards.