Table of Contents
ToggleMedical silicone nasal CPAP masks are essential components of Continuous Positive Airway Pressure (CPAP) therapy, widely used to treat obstructive sleep apnea (OSA) and other respiratory disorders. The performance of a CPAP mask directly affects patient comfort, therapy effectiveness, air leakage control, and long-term compliance.

For manufacturers and OEM buyers, understanding how to produce a medical silicone nasal CPAP mask is critical to meeting medical-grade quality standards, biocompatibility requirements, and regulatory compliance.
What Is a Medical Silicone Nasal CPAP Mask?
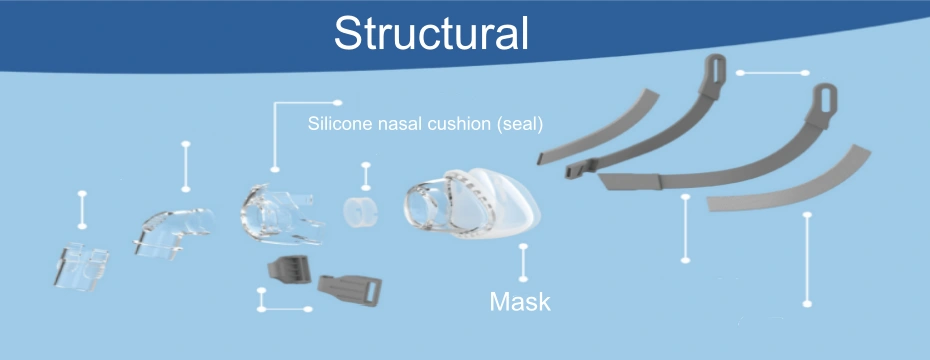
A medical silicone nasal CPAP mask is a patient interface device that delivers pressurized air from a CPAP machine to the patient’s nasal airway during sleep. It typically includes:
- Silicone nasal cushion (seal)
- Mask frame or shell
- Elbow or air inlet connector
- Headgear attachment points
- Optional vent and anti-asphyxia valve
Among all components, the silicone nasal cushion is the most critical, as it determines sealing performance, comfort, and skin safety.
Why Medical Silicone Is Used for CPAP Nasal Masks
1. Superior Biocompatibility
Medical-grade silicone is non-toxic, non-irritating, latex-free, and suitable for prolonged skin contact.
2. Excellent Sealing and Flexibility
Soft silicone adapts to facial contours, reducing air leakage while maintaining comfort.
3. Long-Term Durability
Silicone maintains elasticity after repeated use, cleaning, and disinfection.
4. Chemical and Thermal Stability
Medical silicone resists degradation from oils, sweat, cleaning agents, and temperature changes.

Step 1: Medical Silicone Material Selection
Material selection is the foundation of CPAP mask performance and regulatory compliance.
Liquid Silicone Rubber (LSR)
LSR is the preferred material for producing medical silicone nasal CPAP masks.
Advantages of LSR:
- Platinum-cured, low VOC
- Excellent transparency and color stability
- High precision and repeatability
- Ideal for automated, high-volume medical production
Key Material Requirements
- Medical-grade certification
- ISO 10993 biocompatibility compliance
- Low compression set
- High tear resistance
- Shore hardness typically between 20–40 Shore A
Common Silicone Grades
- Implantable or long-term skin-contact silicone
- Platinum-cured LSR (medical class)
Step 2: Nasal CPAP Mask Product Design
Ergonomic Nasal Cushion Design
- Anatomical nose and facial contours
- Soft sealing lip or dual-wall design
- Even wall thickness to prevent collapse or pressure points
Leak Prevention Design
- Flexible edge geometry
- Adaptive sealing zones
- Optimized cushion rebound properties
Comfort and Patient Compliance
- Lightweight design
- Minimal facial pressure
- Reduced skin marks and irritation
Design for Manufacturability (DFM)
- Suitable draft angles
- Controlled undercuts
- Avoid overly thin or thick sections
- Design compatible with LSR injection molding
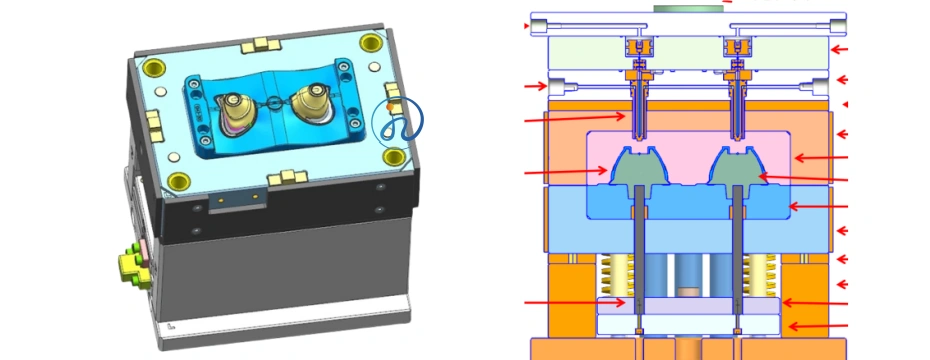
Step 3: Mold Design and Tooling for CPAP Masks
High-precision molds are critical for medical silicone products.
LSR Injection Mold Characteristics
- Cold runner system (standard for LSR)
- Mirror-polished cavity surface
- Precision venting to avoid air traps
- Temperature-controlled mold plates
Multi-Cavity Mold Design
- Improves production efficiency
- Ensures dimensional consistency
- Reduces per-unit manufacturing cost
Mold Materials
- Stainless steel or hardened tool steel
- Corrosion-resistant for medical environments
- Long service life for mass production
Step 4: Liquid Silicone Rubber Injection Molding Process
LSR Injection Molding Overview
LSR injection molding is the most widely used process for producing medical silicone nasal CPAP masks.
Process Steps
- Precise metering of two-part LSR
- Static mixing of components
- Injection into heated mold cavity
- Vulcanization (curing) under controlled temperature
- Automated demolding
- Visual inspection and flash trimming (if necessary)
Why LSR Injection Molding Is Ideal
- High dimensional accuracy
- Minimal material waste
- Clean and contamination-free process
- Suitable for medical cleanroom production

Step 5: Post-Processing and Assembly
Post-Curing
- Removes residual volatiles
- Enhances mechanical and chemical stability
- Often required for medical-grade products
Secondary Operations
- Assembly with mask frame
- Connection of elbow or swivel joints
- Integration with headgear attachment points
- Logo marking or part identification
Cleaning and Sterilization
- Medical-grade washing
- Ultrasonic cleaning
- Optional sterilization (ETO, steam, or gamma, depending on application)
Step 6: Quality Control and Performance Testing
Strict quality control ensures patient safety and therapy effectiveness.
Incoming Material Control
- Certificate of analysis (COA)
- Batch traceability
In-Process Inspection
- Dimensional accuracy checks
- Surface defect inspection
- Flash and consistency control
Functional Testing
- Air leakage testing
- Pressure resistance testing
- Cushion rebound and compression set testing
Biocompatibility and Safety Tests
- Skin irritation and sensitization tests
- Cytotoxicity testing (ISO 10993)

Cost Factors in Producing Medical Silicone Nasal CPAP Masks
Key cost considerations include:
- Medical-grade silicone material
- Mold design complexity
- Cleanroom production requirements
- Assembly and testing processes
- Regulatory and certification costs
Although initial tooling costs can be high, LSR injection molding significantly reduces long-term unit cost for mass production.
Conclusion
Producing a medical silicone nasal CPAP mask requires expertise in medical-grade silicone materials, ergonomic product design, precision LSR injection molding, strict quality control, and regulatory compliance.
For CPAP brands and OEM buyers, partnering with an experienced medical silicone molding manufacturer ensures reliable quality, faster certification, and scalable production.
As demand for sleep apnea therapy continues to grow globally, high-quality silicone nasal CPAP masks remain a core product with long-term market potential.